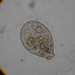
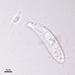
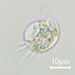
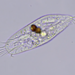
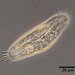
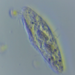
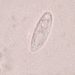
What
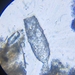
Genus NebelaObserver
nickbelliveauDescription
From mosses and liverworts in damp roadside ditch. 400x magnification.
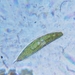
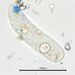
What
Genus NebelaObserver
nickbelliveauDescription
From vernal pool on a bed of Sphagnum sp. 400x magnification.
Photos / Sounds
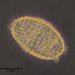
What
Acineria uncinataObserver
peptolabDescription
Acineria uncinata Tucolesco, 1962 from the freshwater pond Kellis Pond which appeared after feeding the culture with boiled wheat seed. Imaged in Nomarski DIC on Olympus BH2 using SPlan 100 1.25 oil objective plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 48 um in length. There are three visible somatic kineties which differentiates this species from A. incurvata which has 11 somatic kineties. Augustin, Foissner & Adam 1987 describe 3 right kineties while Martin-Cereceda, Serrano and Guinea 1995 describe 4 (rarely 5)- 2 left and 2 right. This, and the presence of the short oral slit being restricted to the anterior pole are discriminatory. There are two spherical macronuclei with a large intercalated micronucleus between them.
"Acineria uncinata is a lanceolate ciliate with a short oral slit restricted to the anterior region, and rolled up forming a characteristic overlapping in the anterior area at the left side of the cell. Sizes range between 26-38 um in length and 11-17 um in width in OsO4 fixed specimens. The nuclear apparatus consists of two nearly spherical macronuclei, closely together, and one large micronucleus between them. A single contractile vacuole is at the posterior end of the cell. Extrusomes are regularly placed at the anterior end of the cell, and other are dispersed at the cytoplasm. Somatic infraciliature is composed of four kineties (rarely five) which present a different arrangement at both sides of the cell. The two right ones (rk1, rk2) have long cilia and show kinetodesmal fibers, while the two left somatic kineties (lk1, lk2; a third left kinety is sometimes present) possibly lack such fibers. A kinetal segment with short clavate cilia, composed of pairs of kinetosomes, is located at the left dorsal side. Three perioral kineties are located at both sides of the oral region. Left perioral kinety (pk1) shows kinetosomes with slight fibers which could correspond to transverse microtubules. At right side, two kineties accompany the oral slit, one of them (pk2), short in length, seems to be formed by pairs of kinetosomes whose fibrillar derivates did not stain, while the other kinety (pk3), extending to the posterior end of the cells, is formed by kinetosomes with long cilia and conspicuous kinetodesmal fibers. This perioral kinety is thus very likely a somatic ciliary row except for its anterior region" (1).
"Acineria uncinata Tucolesco, 1962 Brackish and freshwater. About 35-55 um. Body lanceolate without lateral edge. Anterior pole overlapping towards the left side. Two spherical macronuclei showing a clearer zone at their central region. Sometimes a single, elongated, tapered nucleus. Contractile vacuole terminal, often surrounded by a group of smaller vacuoles. Cytostome a straight and short slit restricted to the rolled up anterior pole. Can therefore feed only on small prey (flagellates). Three somatic kineties on the right side with 20-22 cilia each. Cilia at the ventral margin of the anterior third transformed to regularly curved crotchets. Occurence and Ecology. This species was found in summer 1954 in a small dirty brackish puddle near Lake Tekirghiol and in mesosaprobic freshwaters of Bucharest. Tucolesco (1962) separated this species from A. incurvata by the non-overlapping post-oral dorsal margin. However, in A. incurvata the situation is rather similar. Thus, we propose the following characters for discrimination from A. incurvata: the presence of only three somatic kineties on the right side, the (probably) unciliated left side, and the short oral slit being restricted to the anterior pole. Unmentioned since description. Note after proof reading: This is a valid species which we rediscovered recently" (2).
- Mercedes Martin-Cereceda, Susana Serrano, and Almudena Guinea; Occurrence of Acineria uncinata in Activated Sludge Plants: Morphology and Relationship with Physico-Chemical Plant Parameters. Arch.Protistenkd. 146:79-84, 1995
- Revision of the genera Acineria, Trimyema and Trochiliopsis (Protozoa, Ciliophora). H. Augustin, W. Foissner & H. Adam. Bull.Br.Mus.Nat.Hist. (Zool) 1987 52(6): 197-224
Photos / Sounds
What
Nassulopsis elegansObserver
sally3142Description
culture from temporary mud puddles and wet soil with algae in old orchard land W of Tavira, Portugal; collected 3 April 2024
Video on my post to fb group Amateur Microscopy: https://www.facebook.com/1300443920/videos/pcb.2592299670951432/800319331606585
Photos / Sounds
Observer
peptolabDescription
Paramecium nephridiatum V. Gelei, 1925 from the brackish estuarine pond Pussy's Pond, an offshoot of Acabonac Harbor. The population arose after feeding the culture with boiled wheat seeds. Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 40 0.95 and SPlan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
The cells measure from 100-165 um in length. There is a large oval macronucleus usually situated slightly anterior to the cell equator and 3-4 small endosomal-type micronuclei can be observed with exact focus in the anterior end of the cell remote from the macronucleus- an important specific character of P. nephridiatum according to Fokin et al 1999 (1) The oral apparatus is situated posterior to the macronucleus with the cytostome roughly equatorial. There is an inconspicuous tuft of long caudal cilia. The two contractile vacuoles, one anterior and one posterior, have up to 15 short radiating canals and 2-3 excretory pores- another important specific characteristic of P. nephridiatum (1). There is a dense investment of subpellicuar extrusomes. The pellicle is divided into rectangular spaces on optical sectioning of the surface, a feature not mentioned by Fokin et al 1999. These features point strongly to P. nephridiatum. However, the more posterior location of the oral opening (as opposed to slightly anterior to the middle of the cell) and the peculiar pellicular division into rectangular spaces suggest that this might be a cryptic species of P. nephridiatum. Indeed, Przyboś et al 2019 describe 6 cryptic species within P. nephridiatum (2)
The cell shape with its rounded posterior end, and broad anterior part is of the "bursaria" type (Wichterman 1986) or "woodruffi" type according to Jankowski (1969). The oral opening is situated a little anterior to the middle of the cell. The cell surface is uniformly ciliated except for several long caudal cilia located close to the posterior end of the dorsal side, but not on the top. The abundant subpellicular trichocysts are distributed uniformly. Specimens were about 145 x 47 um long in vivo but shrank 10% after the silver nitrate impregnation. In silver-impregnated specimens, there were ~38 rows of ventral kineties and ~35 dorsal kineties. The preoral suture is distinct, but the postoral suture is very obscure. The cytoproct is situated in the posterior third. The vestibular zone is conspicuous and is terminated by the distinctive shape of the buccal opening. Two peniculi and open quadrulus are located on the dorsal and left wall of the buccal cavity. The endoral membrane is situated along the entire right edge of the buccal opening but its dikinetids are not recognizable in all specimens. It has 15 dikinetids on the average. The buccal cavity size various around 30 um. On the dorsal side of the body the contractile vacuole pores are very distinctive both on impregnated specimens as well as in living cells. Usually, both contractile vacuoles have more than one pore each, typically two or three. However, we have found two stocks (WCh-1 and WS-12) where one of the contractile vacuoles quite often (up to 50%) had only one pore. Both contractile vacuoles usually have 8-14 collecting canals, ten on average. Numerous crystals were very often found in the cytoplasm, but their quantity and location varied, probably depending on the culture conditions. The nuclear apparatus is located in the anterior part of the cell. One slightly ellipsoid or ovoid macronucleus, ~30x36 um in living cell and ~17x23 um in stained cells, on average, resides just anterior to the equator of the cell. In Feulgen-stained cells the macronucleus has a very intense colour. The three to four spherical micronuclei of the "endosomal" type (Fokin 1997), ~3 um in diam. (on average) are distributed irregularly along the anterior part of cell. Endocytobiotic bacteria are often found in the cytoplasm (Fokin 1989) and can also be found in the perinuclear space and in the macronucleus (Fokin 1989; Fokin and Sabaneyeva 1997)" (1).
"Swimming behavior - During swimming this species spirals on its long axis in both directions (Fokin 1987). We could not find any simple triggers (food, time) for changing this swimming direction. Typically, "left spiral swimmers" and "right spiral swimmers" were present at the same time in the culture. During the several years of investigation of this trait there was some preference of the cells from the same stocks to spiral in the left direction. Remarks- Gelei (1925) described a new species of Paramecium, Paramecium nephridiatum, based on the material which he had found in his laboratory aquarium. This population was in fact a mixture of the new species and Paramecium caudatum (Gelei 1938). This was the reason why some features of the new ciliate were similar to P. caudatum so that no one recognized this new species in nature, though reference to P. nephridiatum was made by Kahl (1931) and Kalmus (1931). Gelei (1938) redescribed the species from a native population (Tisza River, Szeged, Hungary) using a "clean culture". For unknown reasons, this new description did not attract the attention of protozoologists and in all subsequent reviews, P. nephridiatum was considered a nonvalid species (Vivier 1974; Wichterman 1953, 1986) even when the article of Gelei (1938) was listed in the references. Only once was P. nephridiatum mentioned in a short abstract as a species living in Florida, USA (Bovee 1983), although characters of the species were not listed in this publication. Since 1983, one of us (S. F) has repeatedly collected a species of Paramecium with multiple contactile vacuole pores, which is a distinctive trait of P. nephridiatum, although it was considered for a time as a feature of Paramecium woodruffi (Agamaliev 1983; Fokin 1986), Jankowski (pers. commun.)" (1).
"Occurrence and ecology- A number of stocks of P. nephridiatum were isolated from the sea shores of northern Europe: the North, Baltic, White, and Barents Sea coasts. It was detected during sampling in Woods Hole, MA, USA, Atlantic Ocean and on Sakhalin Island, Sea of Okhotsk. The salinity of these samples varied from 1.5-32 % The species was also found in a fresh-water body in Jerusalem Zoo, Israel. Samples were taken mainly during the summer, from mid-April (Wood Hole) to November (North Sea coast).
Sampling of the same wild population of P. nephridiatum (Sredny Island, White Sea, Russia) has been repeated every year since 1990 to observe long-time changes in the population, as well as the euryhaline ability of the species. This population was present at salinities from 4-35% and in the temperature range from 10-25” C. Very often the populations of P. nephridiatum occurred at the lower limit of oxygen concentrations. They were mainly feeding on bacteria. In the same samples these other ciliates were usually found: Prorodon sp., Frontonia marina, Metopus sp., P. calkinsi, P. woodruffi, and sometimes P. duboscqui" (1).
- Sergei I. Fokin, Thorsten Stoeck, and Helmut J. Schmidt; Rediscovery of Paramecium nephridiatum Gelei, 1925 and its Characteristics. J.Eukaryot.Microbiol. 46(4):416-426, 1999
- Evaluation of the molecular variability and characteristics of Paramecium polycaryum and Paramecium nephridiatum, within subgenus Cypriostomum (Ciliophora, Protista). Ewa Przyboś, Maria Rautian, Alexandra Beliavskaia, Sebastian Tarcz . Mol Phylogenet Evol . 2019 Mar:132:296-306
Photos / Sounds
What
Holosticha pullasterObserver
amayakanDescription
Video: https://youtu.be/yrHLXXZ439Q
Photos are snapshots of the same individual in the video
Cell size: 68 µm in length
Site of collection: Pavilion, Katsurashima Ryokuchi north pond (a freshwater habitat), Sendai, Japan
Date of collection: December 18th 2021
Weather: Snow
Water temp.: 6.6°C
pH 6.5
Date of observation:
December 30th 2021 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
Observer
peptolabDescription
Dexiotricha granulosa Kent 1881 from the freshwater pond Kellis Pond which appeared after feeding the culture with boiled wheat seed. Imaged in Nomarski DIC on Olympus BH2 using SPlan 100 1.25 oil objective plus variable phone camera cropping on Samsung Galaxy S9+. The ciliate measures 36 um in length. The cell is elongate ellipsoid with a long caudal cilium, spherical macronucleus located just posterior to the equator, a similarly positioned posterior central contractile vacuole with collecting vacuoles, and two or three anterior transverse ciliary rows (paratenes). The most striking feature is the presence of numerous ring-shaped granules which are only seen in D. granulosa among others in the genus. Interestingly, Foissner and also Kreutz (realmicrolife.com) describe the mouth of D. granulosa as subapical and small and difficult to detect in vivo; in my population it is located slightly more posteriorly at the end of the anterior 1/3 of the cell and is large and easy to discern with a large undulating membrane.
Bruce Taylor writes: "Dexiotricha granulosa is a scuticociliate, 50-70 μm long, whose cytoplasm is packed with tiny ring-shaped glyocogen granules. The cell has a distinctive pattern of kinetids at the anterior (which looks to me, like the winding path around a tiny mountain). These are not true ciliary rows or kineties but "paratenes", which appear as transverse rows running at right angles to the longitudinal kineties (see Denis Lynn, The Ciliated Protozoa, 2008, p. 43). Contractile vacuole and macronucleus are close together, at the midpoint of the cell.
Dr. Martin Kreutz writes: "The special shape of the storage bodies of this species can already be seen. They are ring-shaped glycogen bodies with a diameter of 2 - 2.5 µm. Most specimens are full of them, making examination of the interior difficult. The mouth opening is also only visible briefly, as Dexiotricha begins to rotate around the longitudinal axis under increasing cover glass pressure and then usually comes to rest on the left or right side. So you have to try to catch the mouth opening of a free-swimming specimen. The slightly triangular shaped mouth opening is surrounded by an undulating membrane and in it there are 3 adoral membranelles, which transport the captured food (bacteria) into the mouth opening. However, these structures are only visible with silver impregnation. On the dorsal side of Dexiotricha granulosa, three transverse rows of cilia are visible, which are very typical for this species. Only in the crushed specimen do the extrusomes become visible, which are distributed over the entire body.
Foissner et al 1994 (1) provide the differential diagnostic features:
Size in vivo 40-80 x 15-30 pm, usually 50-60 pm long.
Shape slender to broadly ovoid and light at the mouth sunken; however, the front end is slightly truncated at an angle
Macronucleus spherical approximately in the middle of the body. 1 spherical micronucleus
Contractile vacuole approximately in the middle of the body
Resting extrusomes (trichocysts) rod-shaped, inconspicuous. Cytoplasm usually densely filled with Approximately 2 pm large, ring-shaped, highly refractive granules, which allows the cell to shine; at low magnification is dark to black.
30-38 longitudinal rows of eyelashes. At the rear there is a rigid caudal cilium about 28 pm long.
Oral apparatus subapical, small and therefore difficult to detect in vivo. 3 small adoral membranelles, to the right of it a semicircular undulating membrane; more detailed structure only visible after silver impregnation, not necessary for determination.
Movement unremarkable, often standing almost still. (1)
"This species is easily recognizable by its numerous ring-shaped granules. Similar granules are present in Loxocephalus ellipticus KAHL, 1931which is probably synonymous with L. kahli TUCOLESCO, 1962a but in this case they are arranged in 5-9 stripes on each side of the body. In Dexiotricha colpidiopsis (KAHL,1926) the contractile vacuole is near the posterior end. Dexioticha polystyla (FOISSNER, 1987) has several caudal cilia (in contrast to the single caudal cilium in D. granulosa). Dexiotricha tranquilla (KAHL, 1926), whose contractile vacuole as in D. granulosa lies approximately in the middle of the body, is smaller (35-60 um) and has only 20-24 rows of cilia (AUGUSTIN & FOISSNER 1992). For identification the characteristics the ring-shaped granules and the ciliature with caudal cilium are most important" (1).
The genus Dexiotricha Stokes, 1885 "was established by Stokes (1885) with the type species Dexiotricha plagia Stokes, 1885, which probably is a junior synonym of D. granulosa (Kent, 1881) Foissner et al., 1994. Jankowski (1964) provided a description of the genus based on live and silverstained specimens from own observations; while more detailed information was given by Jankowski (2007). Dexiotricha had only been recorded from freshwater, soil, and activated sludge, while not from marine or brackish waters. The main distinguishing features of the Dexiotricha species are: 1- the number of somatic and postoral kineties; 2- the number of caudal cilia; and 3- the positions of macronucleus and contractile vacuole (2).
The genus currently comprises seven valid species: Dexiotricha colpidiopsis (Kahl, 1926) Jankowski, 1964; D. elliptica (Kahl, 1931) Fan et al., 2014; D. granulosa (Kent, 1881) Foissner et al. 1994; D. media Peck, 1974); D. polystyla Foissner, 1987; D. raikovi Jankowski, 1964; and D. tranquilla (Kahl, 1926) Augustin & Foissner, 1992" (2).
The large more posterior mouth suggests that this population might be called D. cf granulosa var. macrostoma. There appears to be some variability in populations of D. granulosa. Fan et al 2014 described a species which they called Dexiotricha cf granulosa which had, instead of a central contractile vacuole near the macronucleus, had a subcaudal contractile vacuole (n=5) They wrote: "The species D. granulosa has been numerously reported under several synonyms, and Foissner et al. (1994) synonymized D. plagia and D. media. Considering the great similarities in body size, distinct ring-like granules, the location of the contractile vacuole and the infraciliature, the present study supports the synonymy of the three species. Our population also corresponds well with previous reports on the general infraciliature and most characters from living cells. The prominent difference is the location of the contractile vacuole: in all previous reports, the contractile vacuole of D. granulosa is located equatorially, while, in our population, it is located subcaudally (five individuals in vivo), and the contractile vacuole pore revealed by SEM is also located near the posterior 1/5 (three individuals). Moreover, the number of somatic kineties is fewer than in previous reports (28–30 vs 30–38). The location of the contractile vacuole or excretory pore is considered to be a stable feature and a very important character to distinguish species, and the number of somatic kineties has also been taken into consideration for classification (Fan et al., 2009, 2011a, b; Foissner et al., 1994). However, in this case, considering the similarities of all other characters, especially the general infraciliature and ring-like granules, we are inclined to designate our organism as D. cf. granulosa for the time being. More information based on multiple populations along with sequences of the SSU rDNA are obviously needed" (3).
- Foissner W., Berger H., Kohmann F. (1994) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band III: Hymenostomata, Prostomatida, Nassulida. Informationsber. Bayer. Landesamtes Wasserwirtsch. Heft 1/94: 240-44.
- Redescription of Dexiotricha colpidiopsis (Kahl, 1926) Jankowski, 1964 (Ciliophora, Oligohymenophorea) from a Hot Spring in Iceland with Identification Key for Dexiotricha species. Zhishuai QU, René GROBEN, Viggó MARTEINSSON, Sabine AGATHA, Sabine FILKER, Thorsten STOECK. Acta Protozool. (2018) 57: 95–106
- Morphological reports on two species of Dexiotricha (Ciliophora, Scuticociliatia), with a note on the phylogenetic position of the genus Xinpeng Fan, Saleh A. Al-Farraj, Feng Gao and Fukang Gu. International Journal of Systematic and Evolutionary Microbiology (2014), 64, 680–688
Photos / Sounds
What
Genus CondylostomaObserver
izi_iziDescription
Very very large. Not like anything I have ever seen or can find. Perhaps it is an animal? It really doesn’t look like one
Photos / Sounds
What
Genus PsilotrichaObserver
onotoleDescription
From a moss sample.
Video: https://www.youtube.com/shorts/EQz4QR3D8h8
Photos / Sounds
Observer
peptolabDescription
CHILODONELLA UNCINATA Ehrenberg (Kahl 1931) from the freshwater pond Kellis Pond which appeared after feeding the culture with boiled wheat seed. Imaged in Nomarski DIC on Olympus BH2 using SPlan 100 1.25 oil objective plus variable phone camera cropping on Samsung Galaxy S9+.
The cells measure 40 um in length, a bit smaller than Kahl's described range of 50-90 um. Foissner (1981) givers a range of 28-36 um, Song (1997) around 30 um, and Song et al. (2009) a range of 30-50 um. There are 4 left and 4 right kineties as well as two circumoral kineties and a single preoral kinety. The postoral region is devoid of cilia. The anterior dorsal brush row of bristles is visualized when the indivdual is viewed from the dorsal surface. The macronucleus is ellipsoid and situated posteriorly along with an ovoid micronucleus posterior to the macronucleus. The cyrtos forms a cornucopia-like basket and consists of 10 rods surrounding the circular cytostome. The two contractile vacuoles are diagonally opposed in the anterior right and posterior left quadrants of the cell.
CHILODONELLA UNCINATA Ehrenberg (Kahl, 1931) Length 50-90 um; body dorsoventrally flattened (cr., c. cucullulus); about 11 ventral ciliary rows; dorsally a transverse row of about 7 dorsal cilia or bristles (see Fig. 35B); cytostome round, cytopharyngeal trichites form a cornucopia; macronucleus oval; a single small micronucleus; the 2 contractile vacuoles are located in the anterior right quadrant and the posterior left quadrant, respectively. Chilodonella dentata Fouque is generally held to be a synonym of C. uncinata. Food Bacteria, diatoms, small green algae. Occurrence and ecology Cosmopolitan in distribution, found throughout the year in still and flowing waters (e.g., trickling filters, oxidation ponds). Often numerous in bacterial layers, e.g., at the surface of polluted waters.
CILIATED PROTOZOA. An illustrated guide to the species used as biological indicators in freshwater biology. HARTMUT BICK. WORLD HEALTH ORGANIZATION GENEVA 1972 pp 60-1.
Photos / Sounds
What
Genus PsilotrichaObserver
onotoleDescription
From a moss sample.
Video: https://www.youtube.com/shorts/EQz4QR3D8h8
Photos / Sounds
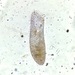
What
Genus StephanopogonObserver
izi_iziDescription
Moved like a ciliate and no flagellum visible. Not a single ciliate ID tool had anything like this. It could be a young euglenoid? I have no idea. Has three “lobes”
Photos / Sounds
What
Stentor muelleriObserver
peptolabDescription
Stentor muelleri EHRENBERG, 1831 from Pussy's Pond, a brackish offshoot of the estuary Acabonac Harbor. Swimming and fully contracted cells. Imaged in Nomarski DIC on Olympus BH2 using SPlanapo 40 0.95 and SPlan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
The extended cells measure from 400 up to 640 um in length. Individuals appear brownish in color and have a moniliform macronucleus composed of a chain of up to 20 ovoid to ellipsoid nodules. I could not observe the lorica since these samples have been disturbed [see discussion below item 8].
" Stentor has a mighty oral appar4tus on the margin of the
broad end. It is cornposed of hundreds of adoral
membranelles (plates of closely spaced cilia acting
together) which are, on the inner side, accompanied by a
single row of cilia, the so-called undulating membrane. The
ciliary plates, which are composed of 25 pm long cilia and
beat 30 times per second, form a spiral band, the so-called
adoral zone of membranelles, which plunges into a conical
buccal cavity leading to the cytostome (mouth proper).The
metachronal beat of the membranelles generates a strong
current swirling food organisms with a velocity of 800 pm s-1 into the buccal cavity" (5).
"Stentor muelleri Ehrenberg, 1831 10-20 macronuclear beads. Slenderly trumpet shaped and 500-1000 um (rarely up to 3 mm) long when fully extended. Mainly in freshwater, but also in estuaries. Recorded from Europe, North America and China . A detailed description and discussion of synonymy, morphology and ecology can be found in Foissner et al. (1992). This freshwater species is well defined by its moniliform macronucleus and the absence of pigmented cortical granules. It has-30-50 somatic kinetics and -10 peristomial ciliary rows. Stentor muelleri has two junior synonyms: S.felici and S.katashimai" (1)
Differential diagnosis from (2)
Elongated individuals in vivo up to 3 mm, usually 500-1000 um long.
Sessile, slim, trumpet-shaped, usually slightly curved. Contracts in an inverted pear shape.
About 10-20 spherical macronucleus parts, which in elongated individuals have a rosary-like form, forming a chain about half the length of the body. 10-17 micronuclei .
Contractile vacuole on the left wall of the oral funnel, with one collecting duct reaching far backwards.
In the plasma and between the cilia, very dense, colorless, granules about 0.5 pm in size. Appears yellowish to brownish at low magnification. No symbiotic algae.
Number of ciliary rows (about 70?) unknown.
The adoral membrane zone extends into the mouth in a spiral to the right. Number of the adoral membranelles unknown. Floor of the mouth with about 20 ciliary rows.
8] Sometimes found with extended, thigmotactic cilia in a 200-1000 um (usually 300-500 um) long, sac-shaped to bottle-shaped, slimy, sometimes very hyaline shell (case usually only noticeable in cells that have been undisturbed for a long time; then often densely covered with detritus).
"Comparisons S. polymorphus and S. coeruleus also have a rosary-shaped macronucleus. The former is colored green by symbiotic algae, the latter has light to strong blue granules between the ciliary rows. Smaller one (500-1200 um) and like S. muelleri uncolored S. roeseli, the macronucleus is rope-shaped. Characteristics 3 are particularly important for identification, 5 important" (2). " Stentor katashimai Kumazawa, 1973 has the main characteristics of s.muelleri, from which it is said to differ by the lack of a lorica, a more stocky shape and the possession of a buccal pouch (Kumazawa, 7974)" (1). "Stentor cornutus Kumazawa 2002 is more conical in shape when extended, while S. muelleri takes a characteristic, slender shape (Kahl, 1935), which is more like an uncoiled trombone (Tartar, 1961a) rather than a trumpet. Stentor cornutus never forms the cylindrical case that S. muelleri usually has when it is attached. The stiff cilia in S. cornutus are shorter, and much less conspicuous than those in S. muelleri. The macronuclear nodes are fewer and more spindle-shaped in S. cornutus than those in S. muelleri, which has more rounded, bead-like nodes" (3).
Fernandes et al 2012 reported a population of Stentor polymorphus devoid of symbiotic algae. They based their diagnosis largely on the absence of loricae in their population and by the presence of a large peristomial pouch (4). The lorica is not always seen in S. muelleri and Foissner et al 1992 attached little importance to its presence (2). The buccal pouch was discounted by Foissner et al 19922 (2) as being a weak character insufficient to discriminate species (2). However Kumazawa 2002 disagrees and stressed presence of the large pouch as an important discriminatory character between their new species S. cornutus as well as S. katashimai (3). Kumazawa 2002, in his figure 1, illustrates the large buccal pouch in S. polymophus (figure 1a) and the lack of same in S. roseli (figure 1b) (3). My observation here has an identical buccal cavity to Kumazawa's figure 1b which he states demonstrates a species lacking the buccal pouch. This serves to differentiate my observation from an aposymbiotic population of S. polymorphus. In addition, the S. polymorphus population of Fernandes et al 2012 had an in vivo length of 850-2000 um, quite a bit larger than my population of S. muelleri at 400-640 um (4).
"Occurrence and distribution: Approximately equally common in stagnant and flowing waters, much rarer than Stentor roeseli. SCHMARDA (1846) found it in all seasons and slow-flowing waters near Vienna. Also in the mesosaprobic samples from Amper near Munich fairly regularly and with a focus on appearing in May/June. We found 1 specimen in the activated sludge. According to ALBRECHT (1984), S. muelleri is oligo- to mesoeuryhaline (see also the evidence from KAHL 1933) in the mouth of the Weser, from BIERNACKA 1962 in the Bay of Danzig, by RIEDEL-LORJE 1981 in alpha to betamesosaprobic samples Elbe Estuary and by JONES 1974 from an estuary in the USA with (0.2-3% salinity). So far only in Europe and Asia (SHEN YUNFEN et at. 1988) and North America" (2).
- Revision of the genus Stentor Oken (Protozoa, Ciliophora) and description of S.araucanus nov. spec. from South American lakes W.Foissner and S.Wölfl. Journal of Plankton Research Vol.l6 no.3 pp.255-289. 1994
- Foissner,W., Berger,H. and Kohmann,F. (1992) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, May 1992, pp. 351-4
- Notes on the taxonomy of Stentor Oken (Protozoa, Ciliophora) and a description of a new species. HIDEO KUMAZAWA. JOURNAL OF PLANKTON RESEARCH VOLUME 24 NUMBER 1 PAGES 69-75 2002
-
Morphology and Phylogenetic Position of an Unusual
Stentor polymorphus (Ciliophora: Heterotrichea) Without Symbiotic Algae. Noemi M. Fernandes, Inacio Domingos da Silva Neto & Carlos G. Schrago. Journal of Eukaryotic Microbiology 2014, 61, 305–312 - Stentor. Wilhelm Foissner. ENCYCLOPEDIA OF LIFE SCIENCES 2002 Macmillan Publishers Ltd, Nature Publishing Group. p 561
Photos / Sounds
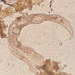
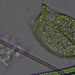
What
Family GonostomidaeObserver
smellyturkeyDescription
Vernal pool. found in sample of this goop: https://www.inaturalist.org/observations/206124448
Photos / Sounds
What
Stentor muelleriObserver
peptolabDescription
Stentor muelleri EHRENBERG, 1831 from Pussy's Pond, a brackish offshoot of the estuary Acabonac Harbor. Imaged in Nomarski DIC on Olympus BH2 using SPlanapo 20 0.70 and SPlanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure from 400 up to 640 um in length. Individuals appear brownish in color and have a moniliform macronucleus composed of a chain of up to 20 ovoid to ellipsoid nodules. I could not observe the lorica since these samples have been disturbed [see discussion below item 8].
"Stentor muelleri Ehrenberg, 1831 10-20 macronuclear beads. Slenderly trumpet shaped and 500-1000 um (rarely up to 3 mm) long when fully extended. Mainly in freshwater, but also in estuaries. Recorded from Europe, North America and China . A detailed description and discussion of synonymy, morphology and ecology can be found in Foissner et al. (1992). This freshwater species is well defined by its moniliform macronucleus and the absence of pigmented cortical granules. It has-30-50 somatic kinetics and -10 peristomial ciliary rows. Stentor muelleri has two junior synonyms: S.felici and S.katashimai" (1)
Differential diagnosis from (2)
1) Elongated individuals in vivo up to 3 mm, usually 500-1000 um long.
2) Sessile, slim, trumpet-shaped, usually slightly curved. Contracts in an inverted pear shape.
3) About 10-20 spherical macronucleus parts, which in elongated individuals have a rosary-like form, forming a chain about half the length of the body. 10-17 micronuclei .
4) Contractile vacuole on the left wall of the oral funnel, with one collecting duct reaching far backwards.
5) In the plasma and between the cilia, very dense, colorless, granules about 0.5 pm in size. Appears yellowish to brownish at low magnification. No symbiotic algae.
6) Number of ciliary rows (about 70?) unknown.
7) The adoral membrane zone extends into the mouth in a spiral to the right. Number of the adoral membranelles unknown. Floor of the mouth with about 20 ciliary rows.
8] Sometimes found with extended, thigmotactic cilia in a 200-1000 um (usually 300-500 um) long, sac-shaped to bottle-shaped, slimy, sometimes very hyaline shell (case usually only noticeable in cells that have been undisturbed for a long time; then often densely covered with detritus).
"Comparisons S. polymorphus and S. coeruleus also have a rosary-shaped macronucleus. The former is colored green by symbiotic algae, the latter has light to strong blue granules between the ciliary rows. Smaller one (500-1200 um) and like S. muelleri uncolored S. roeseli, the macronucleus is rope-shaped. Characteristics 3 are particularly important for identification, 5 important" (2). " Stentor katashimai Kumazawa, 1973 has the main characteristics of s.muelleri, from which it is said to differ by the lack of a lorica, a more stocky shape and the possession of a buccal pouch (Kumazawa, 7974)" (1). "Stentor cornutus Kumazawa 2002 is more conical in shape when extended, while S. muelleri takes a characteristic, slender shape (Kahl, 1935), which is more like an uncoiled trombone (Tartar, 1961a) rather than a trumpet. Stentor cornutus never forms the cylindrical case that S. muelleri usually has when it is attached. The stiff cilia in S. cornutus are shorter, and much less conspicuous than those in S. muelleri. The macronuclear nodes are fewer and more spindle-shaped in S. cornutus than those in S. muelleri, which has more rounded, bead-like nodes" (3).
Fernandes et al 2012 reported a population of Stentor polymorphus devoid of symbiotic algae. They based their diagnosis largely on the absence of loricae in their population and by the presence of a large peristomial pouch (4). The lorica is not always seen in S. muelleri and Foissner et al 1992 attached little importance to its presence (2). The buccal pouch was discounted by Foissner et al 19922 (2) as being a weak character insufficient to discriminate species (2). However Kumazawa 2002 disagrees and stressed presence of the large pouch as an important discriminatory character between their new species S. cornutus as well as S. katashimai (3). Kumazawa 2002, in his figure 1, illustrates the large buccal pouch in S. polymophus (figure 1a) and the lack of same in S. roseli (figure 1b) (3). My observation here has an identical buccal cavity to Kumazawa's figure 1b which he states demonstrates a species lacking the buccal pouch. This serves to differentiate my observation from an aposymbiotic population of S. polymorphus. In addition, the S. polymorphus population of Fernandes et al 2012 had an in vivo length of 850-2000 um, quite a bit larger than my population of S. muelleri at 400-640 um (4).
"Occurrence and distribution: Approximately equally common in stagnant and flowing waters, much rarer than Stentor roeseli. SCHMARDA (1846) found it in all seasons and slow-flowing waters near Vienna. Also in the mesosaprobic samples from Amper near Munich fairly regularly and with a focus on appearing in May/June. We found 1 specimen in the activated sludge. According to ALBRECHT (1984), S. muelleri is oligo- to mesoeuryhaline (see also the evidence from KAHL 1933) in the mouth of the Weser, from BIERNACKA 1962 in the Bay of Danzig, by RIEDEL-LORJE 1981 in alpha to betamesosaprobic samples Elbe Estuary and by JONES 1974 from an estuary in the USA with (0.2-3% salinity). So far only in Europe and Asia (SHEN YUNFEN et at. 1988) and North America" (2).
- Revision of the genus Stentor Oken (Protozoa, Ciliophora) and description of S.araucanus nov. spec. from South American lakes W.Foissner and S.Wölfl. Journal of Plankton Research Vol.l6 no.3 pp.255-289. 1994
- Foissner,W., Berger,H. and Kohmann,F. (1992) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, May 1992, pp. 351-4
- Notes on the taxonomy of Stentor Oken (Protozoa, Ciliophora) and a description of a new species. HIDEO KUMAZAWA. JOURNAL OF PLANKTON RESEARCH VOLUME 24 NUMBER 1 PAGES 69-75 2002
-
Morphology and Phylogenetic Position of an Unusual
Stentor polymorphus (Ciliophora: Heterotrichea) Without Symbiotic Algae. Noemi M. Fernandes, Inacio Domingos da Silva Neto & Carlos G. Schrago. Journal of Eukaryotic Microbiology 2014, 61, 305–312
Photos / Sounds
What
Stentor multiformisObserver
peptolabDescription
Stentor multiformis (Mueller 1786) EHRENBERG, 1838 from Pussy's Pond, a brackish offshoot of the estuary Acabonac Harbor. Imaged in Nomarski DIC on Olympus BH2 using SPlanapo 20 0.70 and SPlanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells have a lovely pale green-blue coloration without symbionts. However, using my Nomarski DIC, I could not bring out the lovely blue-green coloration no matter how I adjusted my DIC slider and the camera settings. I did manage to capture the color under conventional bright field. They are mostly swimming and only rarely are seen to attach to the substrate. The length on the swimming form is 200 um. There is a single spherical macronucleus.
"Stentor multiformis (Müller, 1786) Ehrenberg, 1838 (basionym: Vorticella multiformis). A detailed description and discussion of synonymy, morphology and ecology can be found in Foissner et al. (1992). This is a small, blueish species occurring in marine, freshwater and terrestrial biotopes. It is, however, still uncertain whether the marine and freshwater populations are truly conspecific. In the absence of detailed evidence, we follow Stein (1867) and Kahl (1932), who assume conspecificity. This species is sparingly mentioned in faunal lists, although it is rather frequent (Schuberg, 1896; Foissner et aI.,1992); possibly it has often been mistaken for small individuals of S.coeruleus, Stentor multiformis has 34-45 somatic kineties, 6-9 peristomial ciliary rows and 100-150 adoral membranelles (Packroff and Wilbert, 1991). Two junior synonyms are known: S. gallinulus and S.nanus" (1)
"All evidence before 1900 come from marine biotopes. KAHL 1932 found S. multiformis both in very weak brackish water as well as in the sea. Later it was found in flowing and stagnant waters, i.e. in fresh water repeatedly. Possible confusion- The also blue-green S. coeruleus has a moniliform macronucleus and is much larger (1-2 mm). Features 3 and 5 are particularly important for identification. Always pay attention to the macronucleus" (2).
"Occurrence and distribution: In the benthos and growth (also scum skin) of brackish water ponds among other things, the North and Baltic Seas, the Atlantic and the Pacific (2nd B. BIERNAKA L962, LEVANDER 1901, MÜLLER 1786, STEIN 1867, WAILES 1943). According to KAHL (1933) a typical salt water form. According to the later literature data and our own experiences, also in Lakes and ponds ( BOVEE 1960, GAJEWSKAJA 1933, GRABACKA 1973, PACKROFF & WILBERT 1991, SCHLOTT-IDL 1978, SCHUBERG 1896; own observations from the strong eutrophs Salzburg University Pond and not too rarely in flowing waters (ALBRECHT 1984, PATRICK et al. 1967, REUTER 1963; own observations in the beta to alpha mesosa samples Amper near Munich. PUYTORAC et al. 1972) once found it very often in a small, eutrophic, sphagnum-covered lake near Montreal (Canada). Further evidence in Central Europe European moorland waters: GROLIERE (1977b), PENARD (L922). So far found in Europe, Asia, North and Central America and New Zealand" (2).
"Differential diagnosis
1) Length in vivo 200-500 um, usually around 250 um. Contractile down to 90 um.
2) Trumpet- to obversely pear- or bottle-shaped. Bottom of mouth on the right bulging like a comb.
3) In the middle of the body there is 1 (very rarely 2-4) round to slightly ellipsoid macronucleus, containing many small, flattened micronuclei.
4) Contractile vacuole on the left wall of the oral funnel, with a short anterior collecting duct on the left and a long one posteriorly.
5) Close under the pellicle, between the ciliary rows, very densely packed, azure to sea green granules that form narrow stripes and give the cell a bright appearance at low magnification with strong blue color. No symbiotic algae.
6) 34-45 ciliary rows, the space between which increases in a clockwise direction. Between the short somatic cilia, which can be spread apart, there are also long, stiff tactile bristles.
7) The adoral membrane zone extends into the mouth in a right-handed spiral and consists of from 100-150 adoral membranelles. 6-9 rows of irises on the floor of the mouth. Parallel to the adoral membranelle zone there is an undulating membrane.
8) Lorica housing has so far only been proven by PACKROFF & WILBERT." (2)
- Revision of the genus Stentor Oken (Protozoa, Ciliophora) and description of S.araucanus nov. spec. from South American lakes W.Foissner and S.Wölfl. Journal of Plankton Research Vol.l6 no.3 pp.255-289. 1994
- Foissner,W., Berger,H. and Kohmann,F. (1992) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, May 1992, pp. 351-4
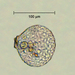
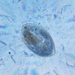
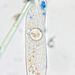
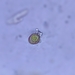
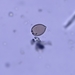
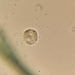
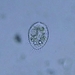
What
Family ArcellidaeObserver
thilokruegerDescription
Found inside trap of Utricularia oppositiflora.
Stats
- 7483